Matter is made up of very tiny particles.
1. Particles of matter are very small and have space between them. – When we make tea, coffee or lemonade (nimbu paani), particles of one type of matter get into the spaces between particles of the other. For example –
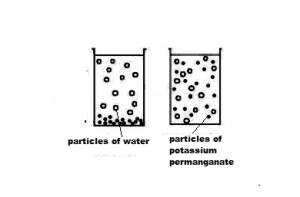
(i) When we dissolve a drop of blue ink or 2-3 crystals of potassium
permanganate (purple, solid) in 100ml of water in a beaker, the colour of water changes into blue / purple itself. This shows that there is enough space between particles of matter.
(ii) Now, we take 10ml of deep purple solution of potassium permanganate in an another beaker and mix it with 90ml of water. The colour of solution will become a bit lighter.

If we keep on diluting the potassium permanganate solution like this a number of times (5-7 times), we find that the colour of water is still coloured but becomes lighter and lighter. Thus, it is concluded that there must be millions of tiny particles in a very small crystal of potassium permanganate or in a small drop of blue ink, which keeps on dividing themselves into smaller and smaller particles as these purple coloured potassium permanganate particles spread throughout the water and make it purple. Similarly, particles of sugar, salt, or dettol get evenly distributed in water.
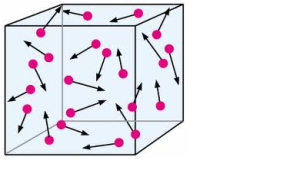
2. Particles of matter are continuously moving –
Particles of matter continuously move in all directions. The random, zig-zag movement of the small particles suspended in a liquid or a gas is called Brownian motion. Any particle in motion, possesses kinetic energy. As the temperature rises, particles move faster. It means, with increase in temperature the kinetic energy of the particles increases.
On burning of an incense stick or agarbatti, the fragrance spreads to every part of the room. Similarly, when we use perfume in a corner of a room, it can be smelled in whole room. The fragrance of an unlit incense stick can be smelled only when it is close to you. However if we lit the incense stick then we can smell the fragrance from anywhere in a room. It concludes –
(i) Particles of matter are continuously moving.
(ii) Initially, the essence of the incense stick is intacked or slow as it is solid in nature but on heating, the movement of particles of matter is fast and it comes out in the gaseous form because we are supplying heat energy to it. As diffusion rate is high in gases, it is spread to larger area and we can smell it from a distance.
The spontaneous migration / spreading out / mixing of a substance into another substance due to the motion of its particles is called diffusion. The smell of hot food or perfume spreads through diffusion. Difffusion is possible as particles of matter are very -very small, have space between them and are always in a state of random motion. The speed of mixing by diffusion depends mainly on three main parameters –
1. temperature
2. size (mass) of the diffusing particles
3. diffusion of the environment
(A) Take two beakers and fill with 100ml cold water in one beaker (X) and 100ml hot water in another beaker (Y). Drop equal size crystals of copper sulphate / potassium permanganate into both the
beakers. We observe that the hot water of beaker (Y) becomes coloured in just a few seconds while in cold water (beaker X) the colour had only spread to some parts of the beaker. This is possible only if we assume that particles of matter move faster in hot water. The temperature of a system is a measure of the average kinetic energy, the energy due to movement, of the particles in the system. Since a higher kinetic energy means a higher velocity, it’s clear why the speed of diffusion increases with
temperature.
(B) Take two beakers and fill with 100ml water in each. Put a drop of red ink in first beaker and equal amount of honey in second beaker. keep them undisturbed. We observe that water in first beaker becomes red uniformly immediately. But in second beaker the honey drop settles down first and takes more time in spreading uniformly. This shows that ink particles being smaller, disperse in a very short time whereas honey particles being bigger and denser take a very long time to disperse in water. Thus, heavy, large particles diffuse more slowly than light, small ones.
(C) Diffusion is most rapid in a gas, slower in a liquid, and very slow or sometimes zero in a solid.
3. Particles of matter attract each other – Particles of matter have force of attraction acting between them and bind them together. The strength of this force of attraction varies from one kind of matter to another.
Consider following three examples to understand how particles of matter attract each other.
(a) Take an iron nail (solid) and try to break it by hammering, cutting or stretching. You will find that it is extremely difficult to break it into pieces. This suggests that particles of iron are bound / attract very strongly to each other.
(b) Take a bucket full of water (liquid). Put a glass rod in it. The glass rod can easily displace water particles. But when you take out the glass rod, water particles again form an undisturbed layer. This concludes that water particles are bound / attract to each other but the attraction is much less as seen in the solid (iron nail). This is the reason how a diver is able to cut through water in a swimming pool. (c) We are surrounded by air (gas). When you throw a flying dish, it flies easily in the air and once the flying dish is passed, the air particles again fill the space immediately. This is only possible when the particles of air attract each other but the attraction is very small.
It concludes –
(i) Particles of all kinds of matter attract each other.
(ii) The strength of the force of attraction (interparticle attraction) varies from one kind of matter to another. In general, the force of attraction is maximum in the particles of solid and minimum in the particles of gas. Thus the increasing order of forces of attraction between the particles is –
Solid > Liquid > Gas





